ICONIC researchers from the Technical University of Munich published a review article reviewing the reaction mechanisms and pathways of the electrochemical nitrate reduction reaction, a key reaction for transforming wastewater nitrate contaminants into valuable and harmless products.
Nitrates and organic nitrogen compounds coming from fertilizers and manure enter groundwater through leaching and reach surface water through runoff from agricultural fields. When nitrates accumulate, the water quality in rivers, lakes, and groundwater is degraded, threatening ecosystems and biodiversity and posing serious health risks to humans.
Although anaerobic bacteria can facilitate nitrate reduction in both terrestrial and marine ecosystems, developing new strategies to reduce and treat nitrate-contaminated soil and water remains crucial and urgent. One of these processes is the electrochemical nitrate reduction reaction (NO₃RR), a low-cost and efficient option for converting nitrate into harmless substances.
Recently, ICONIC researchers at TUM Yixiao Zhang, Qingdian Liao, Elena Gubanova and Aliaksandr Bandarenka published an article in Current Opinion in Electrochemistry reviewing the mechanisms of the reaction, analyzing the recent advancements and the structural and electronic effects of catalysts on the selectivity of products, an essential feature for improving the efficiency and effectiveness of nitrate reduction processes in practical applications.
Challenges in catalysts’ selectivity
The electrochemical nitrate reduction reaction is an important process because it enables the conversion of nitrate ions to less harmful value-added products. However, any electrocatalytic reaction requires a catalyst – a substance that accelerates the chemical reaction under an applied electric potential (voltage), facilitating the transfer of electrons between the electrode and the reactants while enhancing the reaction rate without being consumed in the process. The effectiveness and usefulness of the catalysts vary depending on their type, structure and composition.
Researchers reviewed recent literature on metals such as silver, copper, platinum and cobalt, analyzing how the structure effects – morphology, facets, defects or size -and the electronic effects – modifications, charge distribution and electronic interactions – affect their selectivity and efficiency, impacting their performance.
When looking at copper-based catalysts- one of the most popular choices for the electrocatalytic reduction of nitrate ions- researchers point out that while highly effective, different crystal facets of copper exhibit distinct behaviors, making the specific facet orientation crucial in determining its selectivity. For instance, Cu(100) crystal facet catalysts are highly selective for producing ammonium. Moreover, the surface coverage or structural changes might also alter the catalyst selectivity.
The review also covers single-atom catalysts, which have recently been the focus of advanced research. It points out their promising potential to improve selectivity and efficiency in nitrate reduction. It also highlights the synergistic effects of bimetallic systems, where one metal can stabilize intermediates and lower energy barriers for reactions.
—
Reference: Y. Zhang, Q. Liao, E.L. Gubanova, A.S. Bandarenka. “Relationships among structure, composition, and selectivity in the electrocatalytic reduction of nitrate ions”. Current Opinion in Electrochemistry, Vol. 50. https://doi.org/10.1016/j.coelec.2025.101643
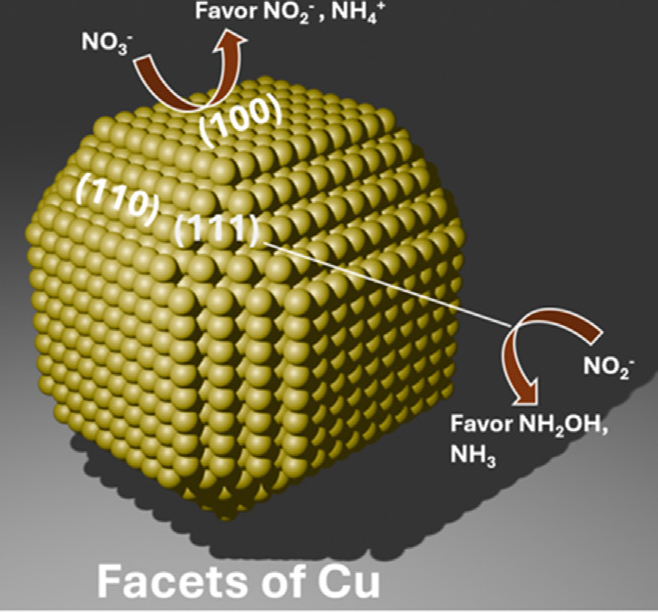
Cu facets showing selectivity for nitrate reduction products. Y. Zhang, Q. Liao, E.L. Gubanova, A.S. Bandarenka. “Relationships among structure, composition, and selectivity in the electrocatalytic reduction of nitrate ions”. Current Opinion in Electrochemistry, Vol. 50.